石墨烯粉體的技術(shù)參數(shù)���。石墨烯粉體按照厚度可分為單層石墨烯,少層石墨烯(2-10個(gè)原子層)和多層石墨烯(又稱為“石墨烯納米片”或“石墨烯微片”)�����;除了厚度���,石墨烯的橫向尺寸也是一個(gè)重要參數(shù)��,不同橫向尺寸石墨烯在原材料選擇�����、工藝過程設(shè)定和工藝設(shè)備開發(fā)方面有不同要求;其外�,石墨烯的純度、均勻性��、N-/P-型摻雜、電導(dǎo)率�、比表面積等參數(shù)也是重要指標(biāo)。
高質(zhì)量石墨烯是指單層或少層石墨烯�,并且層數(shù)均勻。以萬億數(shù)量來計(jì)算的石墨烯片層數(shù)量���,厚度希望全部小于10個(gè)原子層(1克石墨烯���,如果全部1個(gè)原子層,橫向尺寸5微米���,則石墨烯片層數(shù)量約為50萬億片���。單片石墨烯的質(zhì)量計(jì)算公式為:m=2.5g/cm3×0.35nm×5μm×5μm);
高質(zhì)量石墨烯的橫向尺寸分布越窄越好�����;高質(zhì)量石墨烯的純度越高越好�;其外,在分散特性�、表面修飾或摻雜、導(dǎo)電性�����、導(dǎo)熱性、比表面積��、純度等諸多方面要保持較好的特性和一致性���。 在實(shí)際商業(yè)化應(yīng)用中�,石墨烯的品質(zhì)并非越高越好��,需要根據(jù)使用需求進(jìn)行定制開發(fā)�。低成本、批量化���、定制化制備所需要的石墨烯材料是石墨烯走向下游應(yīng)用產(chǎn)品的關(guān)鍵一步�����,但高質(zhì)量石墨烯材料可復(fù)制的���、可規(guī)劃化、可批量化制備技術(shù)依然是石墨烯產(chǎn)業(yè)瓶頸���。
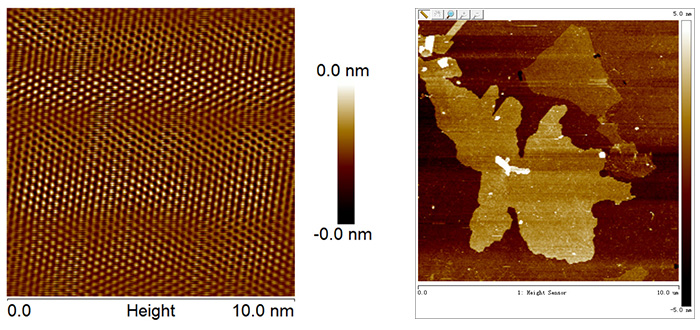
中科悅達(dá)已經(jīng)形成了專業(yè)的科研團(tuán)隊(duì)�,從事石墨烯材料制備工藝的改進(jìn)����、高質(zhì)量石墨烯的創(chuàng)新制備、石墨烯中試和批量化生產(chǎn)技術(shù)開發(fā)�。中科悅達(dá)將與江蘇悅達(dá)新材料科技有限公司聯(lián)手建設(shè)高質(zhì)量氧化還原石墨烯生產(chǎn)線、液相剪切剝離/機(jī)械剝離石墨烯生產(chǎn)線��、液相電化學(xué)石墨烯生產(chǎn)線等�����,為石墨烯下游應(yīng)用產(chǎn)品開發(fā)奠定堅(jiān)實(shí)的基礎(chǔ)�����,并為合作企業(yè)提供可控的石墨烯原材料支持�。
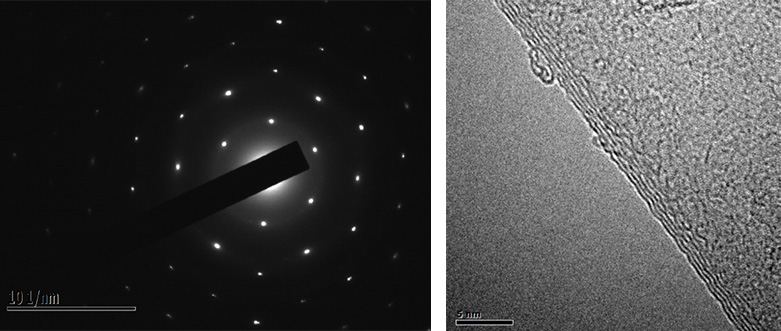
中科悅達(dá)依托中國科學(xué)院上海微系統(tǒng)所的石墨烯粉體科研團(tuán)隊(duì),依托江蘇悅達(dá)集團(tuán)新材料平臺和下游應(yīng)用渠道��,打造頂級的石墨烯材料���。中國科學(xué)院上海微系統(tǒng)所石墨烯粉體團(tuán)隊(duì)���,在丁古巧研究員的帶領(lǐng)下從事石墨烯研究近十年���,探索了氧化還原、液相剝離�、鼓泡剝離等石墨烯材料制備技術(shù)。2018年基于創(chuàng)新的電化學(xué)技術(shù)和超聲輔助分散機(jī)制�����,在NaOH與PTA混合電解液體系中實(shí)現(xiàn)了少層高濃度水溶性石墨烯的制備(Green Chemistry, 2018, DOI:10.1039/C7GC03345A)�����。在這一體系中��,研究人員通過控制電化學(xué)過程����,使PTA析出并吸附于石墨電極,促進(jìn)石墨充分氧化和逐層剝離��,隨后輔以超聲處理進(jìn)一步提高產(chǎn)率�,實(shí)現(xiàn)了高產(chǎn)率(87.3 %)、高固含量(8.2 g/L)以及高穩(wěn)定性的少層微米尺寸水溶性石墨烯的制備���,相對于已有研究報(bào)道具有明顯優(yōu)勢���。同時(shí)����,由于電化學(xué)陽極分子吸附保護(hù)機(jī)制���,實(shí)現(xiàn)了產(chǎn)物片層sp2?結(jié)構(gòu)的保留,制備成膜后��,經(jīng)較低溫度熱還原便可獲得較高的電導(dǎo)率(9517 S/m)��,應(yīng)用潛力巨大���。進(jìn)一步的機(jī)理研究明確了不同于以往插層�、氧化���、膨脹����、剝離的電化學(xué)機(jī)制���,逐層剝離和深入氧化的電化學(xué)新機(jī)制克服了傳統(tǒng)電化學(xué)方法剝離不完全導(dǎo)致的產(chǎn)物層數(shù)多���、質(zhì)量低�、分散性差等問題����。同時(shí),進(jìn)一步的研究確定了超聲在不同環(huán)境���、不同處理時(shí)間及不同前驅(qū)體條件下的效果�,對于后續(xù)研究中的工藝改進(jìn)具有重要指導(dǎo)意義���。所制得的水溶性石墨烯極易成膜��,在電熱方面表現(xiàn)出低電壓���、高速升溫和溫度一致性好等優(yōu)點(diǎn),有望作為新型電熱材料推廣應(yīng)用����。
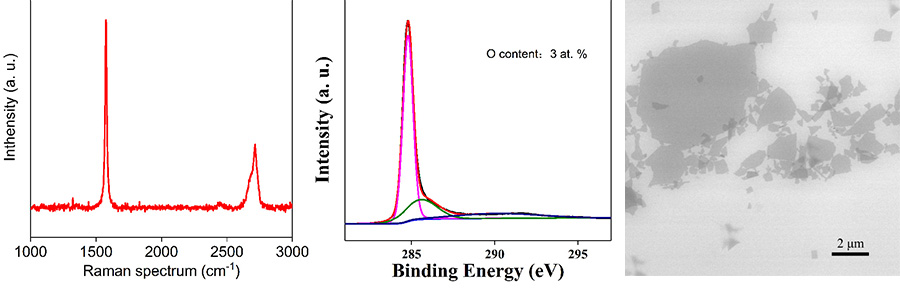
2017年,中國科學(xué)院上海微系統(tǒng)所丁古巧等研究人員在高質(zhì)量石墨烯材料制備方面取得的兩項(xiàng)重要研究進(jìn)展:基于插層-剝離體系的創(chuàng)新和過程控制�,分別在H2SO4-Na2S2O8?和H2C2O4-H2O2?體系中通過化學(xué)(Chemistry of Materials 29, 2017, 8578)和電化學(xué)(Chemistry of Materials 29, 2017, 6214)技術(shù)獲得高質(zhì)量少層石墨烯。在H2SO4-Na2S2O8?體系中,研發(fā)人員通過插層和剝離的動(dòng)力學(xué)控制�,實(shí)現(xiàn)了90.8%少層石墨烯的超高產(chǎn)率,相對于已有報(bào)道具有明顯優(yōu)勢���,所得高質(zhì)量少層石墨烯具有大片徑(平均片徑面積達(dá)54.7 μm2)和高電導(dǎo)率(1.01×105?S m-1)等特點(diǎn)�,在多個(gè)領(lǐng)域應(yīng)用潛力巨大�����。詳盡的機(jī)理研究明確了低溫化學(xué)插層和高溫氣泡剝離的必要性和優(yōu)越性��,深化了對氣體鼓泡剝離制備高質(zhì)量石墨烯過程的理解����,該研究成果對于濕法化學(xué)規(guī)?����;苽涓哔|(zhì)量石墨烯具有重要借鑒意義����。同樣利用氣泡剝離制備高質(zhì)量石墨烯,團(tuán)隊(duì)設(shè)計(jì)開發(fā)的H2C2O4-H2O2?綠色電解液體系���,克服了常規(guī)電化學(xué)方法所得石墨烯氧化缺陷多����、制備過程釋放有害氣體等缺點(diǎn),所獲得的石墨烯含氧量僅有2.41 at. %�����。機(jī)理研究表明��,H2C2O4?不僅是綠色的插層劑���,還能有效阻礙石墨烯在陽極發(fā)生的氧化過程�,而H2O2?對增強(qiáng)氣泡剝離效果明顯�����,該研究成果為開發(fā)電化學(xué)法規(guī)?��;苽涓哔|(zhì)量石墨烯提供了新思路���。
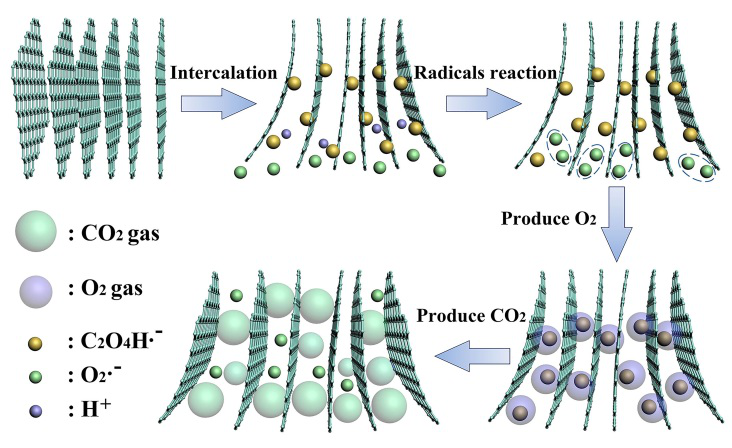
圖:草酸-雙氧水體系中的電化學(xué)剝離
?
中國科學(xué)院上海微系統(tǒng)所在高質(zhì)量石墨烯制備和應(yīng)用方面的代表性研究成果:
1.?Phase Separation Induced PVDF/Graphene Coating on Fabrics towards Flexible Piezoelectric Sensors, ACS Applied Materials & Interfaces 10 (2018)30732-30740.
2.?Anode coverage for enhanced electrochemical oxidation: a green and efficient strategy towards water-dispersible graphene, Green Chemistry 20 (2018)1306-1315.
3.?Three-dimensional cross-linking composite of graphene, carbon nanotube and Si nanoparticles for lithium ion battery anode, Nanotechnology?29 (2018) 125603.
4.?C3N - a 2D crystalline, hole-free, tunable-narrow-bandgap semiconductor with high on-off current ratio and ferromagnetic properties, Advanced Materials?29 (2017) 1605625.
5.?Kinetically enhanced bubble-exfoliation of graphite towards high-yield preparation of high-quality graphene,?Chemistry of Materials?29 (2017) 8578-8582.
6.?Electrochemical Fabrication of High Quality Graphene in Mixed Electrolyte for Ultrafast Electrothermal Heater, Chemistry of Materials?29 (2017) 6214-6219.
7.?Green and Mild Oxidation: An Efficient Strategy towards Water-Dispersible Graphene, ACS Applied Materials & Interfaces?9 (2017) 2856-2866.
8.?One-step Fast Electrochemical Fabrication of Water-dispersible Graphene, Carbon, 111 (2017) 617-621.
9.?Variability of graphene devices?fabricated using graphene inks: atomic force microscope tips, Surface & Coating Technology?320 (2017) 391-395.
10.?A New Graphene Derivative: Hydroxylated Graphene with Excellent Biocompatibility, ACS Applied Materials & Interfaces?8 (2016) 10226-10233. ?
11.?Controllable Edge Oxidation and Bubbling Exfoliation Enable the Fabrication of High Quality Water Dispersible Graphene, Scientific Reports, 6 (2016) 34127.???
12.?Ultrafast adsorption and selective desorption of aqueous aromatic dyes by graphene sheets modified by graphene quantum dots, Nanotechnology?24 (2016) 245703.
13.?Processable aqueous?dispersions of graphene stabilized by graphene quantum dots, Chemistry of Materials 27 (2015) 218-226.
14.?Urea-assisted aqueous exfoliation of graphite for high-quality graphene, Chemical Communications 51 (2015) 4651-4654
15.?Facile Thermal Annealing of Graphite Oxide in Air for Graphene with Higher C/O Ratios, RSC Advances?5 (2015) 69854-69860. ?
16.??Tungsten?oxide nanowire-reduced?graphene?oxide aerogel for high-efficiency visible light photocatalyst, Carbon?78 (2014) 38.
17.?Raman enhancement by graphene-Ga2O3?2D bilayer film, Nanoscale Research Letters?9 (2014) 48.?
18.?Enhanced electromagnetic wave absorption performances of Co3O4?nanocube/reduced graphene oxide composite, Synthetic Metals?194 (2014) 52.
19.?Chemical vapor deposition of graphene on liquid metal catalysts,?Carbon?53 (2013) 321-326.?
20.?The Preparation and Characterization of graphene oxide/poly(vinyl alcohol) composite nanofibers via electrospinning, Journal of Applied Polymer Science 127 (2013) 3026-3032.?
聯(lián)系方式:182-2122-5021(周經(jīng)理)